- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Why does lithium battery capacity decrease in winter
Why does lithium battery capacity decrease in winter
冬天为什么锂电池容量会变低
Since entering the market, lithium-ion batteries have been widely used due to their advantages such as long lifespan, large specific capacity, and no memory effect. Low temperature use of lithium-ion batteries has problems such as low capacity, severe attenuation, poor cycle rate performance, obvious lithium evolution, and imbalanced lithium removal and insertion. However, with the continuous expansion of application fields, the constraints brought by the poor low-temperature performance of lithium-ion batteries are becoming increasingly apparent.
锂离子电池自从进入市场以来,以其寿命长、比容量大、无记忆效应等优点,获得了广泛的应用。锂离子电池低温使用存在容量低、衰减严重、循环倍率性能差、析锂现象明显、脱嵌锂不平衡等问题。然而,随着应用领域不断拓展,锂离子电池的低温性能低劣带来的制约愈加明显。
According to reports, the discharge capacity of lithium-ion batteries at -20 ℃ is only about 31.5% of that at room temperature. Traditional lithium-ion batteries operate at temperatures between -20~+55 ℃. However, in fields such as aerospace, military, and electric vehicles, it is required that the battery can operate normally at -40 ℃. Therefore, improving the low-temperature properties of lithium-ion batteries is of great significance.
据报道,在-20℃时锂离子电池放电容量只有室温时的31.5%左右。传统锂离子电池工作温度在-20~+55℃之间。但是在航空航天、军工、电动车等领域,要求电池能在-40℃正常工作。因此,改善锂离子电池低温性质具有重大意义。
制约锂离子电池低温性能的因素
- In low-temperature environments, the viscosity of the electrolyte increases and even partially solidifies, leading to a decrease in the conductivity of lithium-ion batteries.
-
低温环境下,电解液的黏度增大,甚至部分凝固,导致锂离子电池的导电率下降。
- The compatibility between electrolyte, negative electrode, and separator deteriorates in low-temperature environments.
-
低温环境下电解液与负极、隔膜之间的相容性变差。
- The negative electrode of lithium-ion batteries in low-temperature environments experiences severe lithium precipitation, and the precipitated metallic lithium reacts with the electrolyte, resulting in the deposition of its products and an increase in the thickness of the solid electrolyte interface (SEI).
-
低温环境下锂离子电池的负极析出锂严重,并且析出的金属锂与电解液反应,其产物沉积导致固态电解质界面(SEI)厚度增加。
- In low-temperature environments, the diffusion system of lithium-ion batteries within the active material decreases, and the charge transfer impedance (Rct) significantly increases.
-
低温环境下锂离子电池在活性物质内部扩散系统降低,电荷转移阻抗(Rct)显著增大。
对于影响锂离子电池低温性能因素的探讨
Expert Opinion 1: Electrolyte has the greatest impact on the low-temperature performance of lithium-ion batteries, and the composition and physicochemical properties of electrolyte have an important impact on the low-temperature performance of batteries. The problem faced by low-temperature cycling of batteries is that the viscosity of the electrolyte increases, the ion conduction speed slows down, and the migration speed of electrons in the external circuit does not match, resulting in severe polarization of the battery and a sharp decrease in charging and discharging capacity. Especially when charging at low temperatures, lithium ions can easily form lithium dendrites on the negative electrode surface, leading to battery failure.
专家观点一:电解液对锂离子电池低温性能的影响最大,电解液的成分及物化性能对电池低温性能有重要影响。电池低温下循环面临的问题是:电解液粘度会变大,离子传导速度变慢,造成外电路电子迁移速度不匹配,因此电池出现严重极化,充放电容量出现急剧降低。尤其当低温充电时,锂离子很容易在负极表面形成锂枝晶,导致电池失效。
The low-temperature performance of an electrolyte is closely related to its own conductivity. Electrolytes with high conductivity transport ions quickly and can exert more capacity at low temperatures. The more lithium salts dissociate in the electrolyte, the more migration occurs, and the higher the conductivity. The higher the conductivity and the faster the ion conduction rate, the smaller the polarization received, and the better the performance of the battery at low temperatures. Therefore, a higher conductivity is a necessary condition for achieving good low-temperature performance of lithium-ion batteries.
电解液的低温性能与电解液自身电导率的大小关系密切,电导率大电解液的传输离子快,低温下可以发挥出更多的容量。电解液中的锂盐解离的越多,迁移数目就越多,电导率就越高。电导率高,离子传导速率越快,所受极化就越小,在低温下电池的性能表现越好。因此较高的电导率是实现锂离子蓄电池良好低温性能的必要条件。
The conductivity of an electrolyte is related to its composition, and reducing the viscosity of the solvent is one of the ways to improve the conductivity of the electrolyte. The good fluidity of solvents at low temperatures is a guarantee for ion transport, and the solid electrolyte film formed by the electrolyte on the negative electrode at low temperatures is also a key factor affecting lithium ion conduction, and RSEI is the main impedance of lithium-ion batteries in low-temperature environments.
电解液的电导率与电解液的组成成分有关,减小溶剂的粘度是提高电解液电导率的途径之一。溶剂低温下溶剂良好的流动性是离子运输的保障,而低温下电解液在负极所形成的固体电解质膜也是影响锂离子传导的关键,且RSEI为锂离子电池在低温环境下的主要阻抗。
Expert 2: The main factor limiting the low-temperature performance of lithium-ion batteries is the rapidly increasing Li+diffusion impedance at low temperatures, rather than the SEI membrane.
专家二:限制锂离子电池低温性能的主要因素是低温下急剧增加的Li+扩散阻抗,而并非SEI膜。
Low temperature characteristics of positive electrode materials for lithium-ion batteries
锂离子电池正极材料的低温特性
1. Low temperature characteristics of layered positive electrode materials
1、层状结构正极材料的低温特性
Layered structure, with unparalleled rate performance compared to one-dimensional lithium-ion diffusion channels and structural stability of three-dimensional channels, is the earliest commercially available positive electrode material for lithium-ion batteries. Its representative substances include LiCoO2, Li (Co1 xNix) O2, and Li (Ni, Co, Mn) O2.
层状结构,既拥有一维锂离子扩散通道所不可比拟的倍率性能,又拥有三维通道的结构稳定性,是最早商用的锂离子电池正极材料。其代表性物质有LiCoO2、Li(Co1-xNix)O2和Li(Ni,Co,Mn)O2等。
Xie Xiaohua et al. studied LiCoO2/MCMB and tested its low-temperature charging and discharging characteristics.
谢晓华等以LiCoO2/MCMB为研究对象,测试了其低温充放电特性。
The results showed that as the temperature decreased, the discharge plateau decreased from 3.762V (0 ℃) to 3.207V (-30 ℃); The total battery capacity has also sharply decreased from 78.98mA · h (0 ℃) to 68.55mA · h (-30 ℃).
结果显示,随着温度的降低,其放电平台由3.762V(0℃)下降到3.207V(–30℃);其电池总容量也由78.98mA·h(0℃)锐减到68.55mA·h(–30℃)。
2. Low temperature characteristics of spinel structured cathode materials
2、尖晶石结构正极材料的低温特性
The spinel structured LiMn2O4 cathode material has the advantages of low cost and non toxicity due to its absence of Co element.
尖晶石结构LiMn2O4正极材料,由于不含Co元素,故而具有成本低、无毒性的优势。
However, the variable valence states of Mn and the Jahn Teller effect of Mn3+result in structural instability and poor reversibility of this component.
然而,Mn价态多变和Mn3+的Jahn-Teller效应,导致该组分存在着结构不稳定和可逆性差等问题。
Peng Zhengshun et al. pointed out that different preparation methods have a great impact on the electrochemical performance of LiMn2O4 cathode materials. Take Rct as an example: the Rct of LiMn2O4 synthesized by high-temperature solid phase method is significantly higher than that synthesized by sol gel method, and this phenomenon is also reflected in the lithium ion diffusion coefficient. The main reason for this is that different synthesis methods have a significant impact on the crystallinity and morphology of the products.
彭正顺等指出,不同制备方法对LiMn2O4正极材料的电化学性能影响较大,以Rct为例:高温固相法合成的LiMn2O4的Rct明显高于溶胶凝胶法合成的,且这一现象在锂离子扩散系数上也有所体现。究其原因,主要是由于不同合成方法对产物结晶度和形貌影响较大。
3. Low temperature characteristics of phosphate system cathode materials
3、磷酸盐体系正极材料的低温特性
LiFePO4, together with ternary materials, has become the main positive electrode material for power batteries due to its excellent volume stability and safety.
尖晶石结构LiMn2O4正极材料,由于不含Co元素,故而具有成本低、无毒性的优势。
The poor low-temperature performance of lithium iron phosphate is mainly due to its material being an insulator, low electronic conductivity, poor lithium ion diffusion, and poor conductivity at low temperatures, which increases the internal resistance of the battery and is greatly affected by polarization, hindering the charging and discharging of the battery, resulting in unsatisfactory low-temperature performance.
LiFePO4因绝佳的体积稳定性和安全性,和三元材料一起,成为目前动力电池正极材料的主体。磷酸铁锂低温性能差主要是因为其材料本身为绝缘体,电子导电率低,锂离子扩散性差,低温下导电性差,使得电池内阻增加,所受极化影响大,电池充放电受阻,因此低温性能不理想。
When studying the charge and discharge behavior of LiFePO4 at low temperatures, Gu Yijie et al. found that its Coulombic efficiency decreased from 100% at 55 ℃ to 96% at 0 ℃ and 64% at -20 ℃, respectively; The discharge voltage decreases from 3.11V at 55 ℃ to 2.62V at -20 ℃.
谷亦杰等在研究低温下LiFePO4的充放电行为时发现,其库伦效率从55℃的100%分别下降到0℃时的96%和–20℃时的64%;放电电压从55℃时的3.11V递减到–20℃时的2.62V。
Xing et al. modified LiFePO4 using nanocarbon and found that the addition of nanocarbon conductive agents reduced the sensitivity of LiFePO4's electrochemical performance to temperature and improved its low-temperature performance; The discharge voltage of modified LiFePO4 decreased from 3.40V at 25 ℃ to 3.09V at -25 ℃, with a decrease of only 9.12%; And its battery efficiency is 57.3% at -25 ℃, higher than 53.4% without nanocarbon conductive agents.
Xing等利用纳米碳对LiFePO4进行改性,发现,添加纳米碳导电剂后,LiFePO4的电化学性能对温度的敏感性降低,低温性能得到改善;改性后LiFePO4的放电电压从25℃时的3.40V下降到–25℃时的3.09V,降低幅度仅为9.12%;且其在–25℃时电池效率为57.3%,高于不含纳米碳导电剂的53.4%。
Recently, LiMnPO4 has aroused strong interest among people. Research has found that LiMnPO4 has advantages such as high potential (4.1V), no pollution, low price, and large specific capacity (170mAh/g). However, due to the lower ionic conductivity of LiMnPO4 compared to LiFePO4, Fe is often used to partially replace Mn to form LiMn0.8Fe0.2PO4 solid solutions in practice.
近来,LiMnPO4引起了人们浓厚的兴趣。研究发现,LiMnPO4具有高电位(4.1V)、无污染、价格低、比容量大(170mAh/g)等优点。然而,由于LiMnPO4比LiFePO4更低的离子电导率,故在实际中常常利用Fe部分取代Mn形成LiMn0.8Fe0.2PO4固溶体。
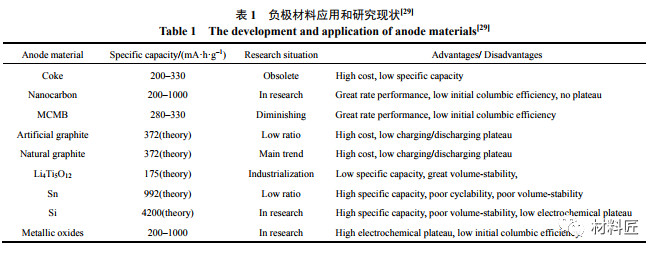
锂离子电池负极材料的低温特性
Compared to positive electrode materials, the low-temperature degradation phenomenon of negative electrode materials in lithium-ion batteries is more severe, mainly due to the following three reasons:
相对于正极材料而言,锂离子电池负极材料的低温恶化现象更为严重,主要有以下3个原因:
- During low-temperature high rate charging and discharging, the battery polarization is severe, and a large amount of lithium metal deposits on the negative electrode surface, and the reaction products between lithium metal and electrolyte generally do not have conductivity;
-
低温大倍率充放电时电池极化严重,负极表面金属锂大量沉积,且金属锂与电解液的反应产物一般不具有导电性;
- From a thermodynamic perspective, the electrolyte contains a large number of polar groups such as C-O and C-N, which can react with negative electrode materials, resulting in SEI films that are more susceptible to low temperature effects;
-
从热力学角度,电解液中含有大量 C–O、C–N 等极性基团,能与负极材料反应,所形成的 SEI 膜更易受低温影响;
- It is difficult to embed lithium in carbon negative electrodes at low temperatures, resulting in asymmetric charging and discharging.
-
碳负极在低温下嵌锂困难,存在充放电不对称性。
低温电解液的研究
The electrolyte plays a role in transmitting Li+in lithium-ion batteries, and its ion conductivity and SEI film formation performance have a significant impact on the low-temperature performance of the battery. There are three main indicators for judging the quality of low-temperature electrolytes: ion conductivity, electrochemical window, and electrode reaction activity. The level of these three indicators largely depends on their constituent materials: solvents, electrolytes (lithium salts), and additives. Therefore, the study of the low-temperature performance of various parts of the electrolyte is of great significance for understanding and improving the low-temperature performance of batteries.
电解液在锂离子电池中承担着传递 Li+ 的作用,其离子电导率和 SEI 成膜性能对电池低温性能影响显著。判断低温用电解液优劣,有3个主要指标:离子电导率、电化学窗口和电极反应活性。而这3个指标的水平,在很大程度上取决于其组成材料:溶剂、电解质(锂盐)、添加剂。因此,电解液的各部分低温性能的研究,对理解和改善电池的低温性能,具有重要的意义。
- Compared to chain carbonates, EC based electrolytes have a compact structure, high interaction force, and higher melting point and viscosity. However, the large polarity brought by the circular structure often results in a high dielectric constant. The high dielectric constant, high ion conductivity, and excellent film-forming performance of EC solvents effectively prevent the co insertion of solvent molecules, making them indispensable. Therefore, most commonly used low-temperature electrolyte systems are based on EC and mixed with low melting point small molecule solvents.
-
EC 基电解液低温特性相比链状碳酸酯而言,环状碳酸酯结构紧密、作用力大,具有较高的熔点和黏度。但是、环状结构带来的大的极性,使其往往具有很大的介电常数。EC 溶剂的大介电常数、高离子导电率、绝佳成膜性能,有效防止溶剂分子共插入,使其具有不可或缺的地位,所以,常用低温电解液体系大都以EC为基,再混合低熔点的小分子溶剂。
- Lithium salts are an important component of electrolytes. Lithium salts in electrolytes can not only improve the ionic conductivity of the solution, but also reduce the diffusion distance of Li+in the solution. Generally speaking, the higher the concentration of Li+in a solution, the higher its ion conductivity. However, the concentration of lithium ions in the electrolyte is not linearly correlated with the concentration of lithium salts, but rather exhibits a parabolic shape. This is because the concentration of lithium ions in the solvent depends on the strength of the dissociation and association of lithium salts in the solvent.
-
锂盐是电解液的重要组成。锂盐在电解液中不 仅能够提高溶液的离子电导率,还能降低 Li+ 在溶液中的扩散距离。一般而言,溶液中的Li+浓度越大,其离子电导率也越大。但电解液中的锂离子浓度与锂盐的浓度并非呈线性相关,而是呈抛物线状。这是因为,溶剂中锂离子浓度取决于锂盐在溶剂中的离解作用和缔合作用的强弱。
Research on Low Temperature Electrolytes
低温电解液的研究
In addition to the battery composition itself, process factors in practical operation can also have a significant impact on battery performance.
除电池组成本身外,在实际操作中的工艺因素, 也会对电池性能产生很大影响。
(1) Preparation process. Yaqub et al. studied the effect of electrode load and coating thickness on the low-temperature performance of LiNi0.6Co0.2Mn0.2O2/Graphite batteries and found that in terms of capacity retention, the smaller the electrode load and the thinner the coating layer, the better its low-temperature performance.
(1) 制备工艺。Yaqub 等研究了电极荷载及 涂覆厚度对 LiNi0.6Co0.2Mn0.2O2 /Graphite 电池低温性能的影响发现,就容量保持率而言,电极荷载 越小,涂覆层越薄,其低温性能越好。
(2) Charging and discharging status. Petzl et al. studied the effect of low-temperature charging and discharging conditions on the cycle life of batteries and found that when the discharge depth is large, it will cause significant capacity loss and reduce the cycle life.
(2) 充放电状态。Petzl 等研究了低温充放电 状态对电池循环寿命的影响,发现,放电深度较大时,会引起较大的容量损失,且降低循环寿命。
(3) Other factors. The surface area, pore size, electrode density, wettability between electrode and electrolyte, and separator all affect the low-temperature performance of lithium-ion batteries. In addition, the impact of material and process defects on the low-temperature performance of batteries cannot be ignored.
(3) 其它因素。电极的表面积、孔径、电极密度、电极与电解液的润湿性及隔膜等,均影响着锂离子电池的低温性能。另外,材料和工艺的缺陷对电池低温性能的影响也不容忽视。
Summary
总结
To ensure the low-temperature performance of lithium-ion batteries, the following points need to be done well:
(1) Forming a thin and dense SEI film;
(2) Ensure that Li+has a high diffusion coefficient in the active substance;
(3) Electrolytes have high ionic conductivity at low temperatures.
In addition, research can take a different approach and focus on another type of lithium-ion battery - all solid state lithium-ion batteries. Compared to conventional lithium-ion batteries, all solid-state lithium-ion batteries, especially all solid-state thin-film lithium-ion batteries, are expected to completely solve the capacity degradation and cycling safety issues of batteries used at low temperatures.
为保证锂离子电池的低温性能,需要做好以下几点:
(1) 形成薄而致密的 SEI 膜;
(2) 保证 Li+ 在活性物质中具有较大的扩散系数;
(3) 电解液在低温下具有高的离子电导率。
此外,研究中还可另辟蹊径,将目光投向另一类锂离子电池——全固态锂离子电池。相较常规的锂离子电池而言,全固态锂离子电池,尤其是全固态薄膜锂离子电池,有望彻底解决电池在低温下使用的容量衰减问题和循环安全问题。



